-
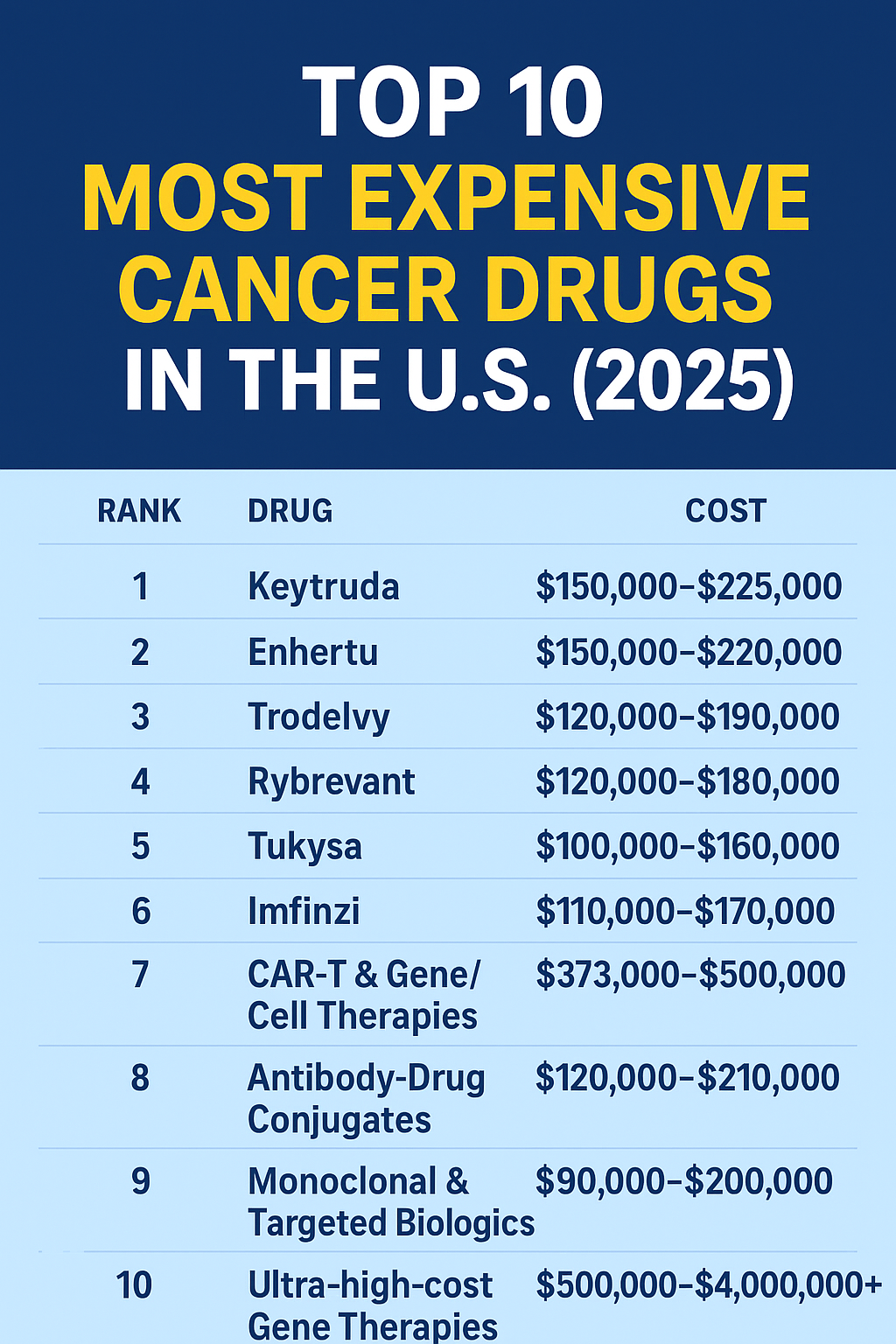
The list price for commercially approved CAR-T products in the U.S. generally ranges from ~$373,000 to $537,000 per infusion depending on the product and indication. These figures refer to the manufacturer list price for the cell product and do not include hospital, ICU, or complication management costs. Yahoo Finance+1
-
Total treatment costs (drug + hospitalization + ICU + supportive care) commonly exceed $500,000 and can approach $1,000,000 in complex cases. Medicare inpatient claims data show wide ranges (median and mean costs vary by product and setting). PMC+1
Why CAR-T is expensive (short explainer)
CAR-T is a personalized, one-time cell therapy that requires harvesting a patient’s T cells, shipping them to a GMP manufacturing site, genetically engineering and expanding them, testing, shipping back, and hospital administration with specialized staff and monitoring (for cytokine release syndrome, neurotoxicity, etc.). Those manufacturing and care costs drive the high price. /+1
2026 U.S. Price Table — Manufacturer list prices and typical total cost ranges
Note: “List price” = manufacturer’s published price for the CAR-T product. “Typical total cost” = manufacturer price + hospital/administration + common complication management (estimates from Medicare/claims analyses and major health reporting). Actual patient bills depend on insurance, negotiated hospital rates, and clinical course.
| Product (brand / generic) | Typical manufacturer list price (U.S.) | Typical total treatment cost range (drug + hospital & care) | Key indications |
|---|---|---|---|
| Kymriah (tisagenlecleucel) | $373,000 – $475,000 depending on indication. (ALL vs DLBCL historic prices). BioInformant+1 | $500,000 – $800,000+ (with hospital & complications). / | Pediatric ALL; certain lymphomas |
| Yescarta (axicabtagene ciloleucel) | ~$373,000 – $537,500 (product/regimen and reporting varies). Reuters+1 | $500,000 – $900,000+ (claims show median total costs ~ $600k). Medical News Today+1 | Large B-cell lymphoma, etc. |
| Tecartus (brexucabtagene autoleucel) | ~$373,000 – $420,000+ (varies by indication). Reuters | $500,000 – $900,000 | Mantle cell lymphoma, certain leukemias |
| Breyanzi (lisocabtagene maraleucel) | ~$375,000 – $420,000 (list prices reported / updated). Department of Financial Services | $500,000 – $800,000 | Relapsed/refractory large B-cell lymphoma |
| Abecma (idecabtagene vicleucel) | ~$400,000+ (multiple myeloma CAR-T; price updates vary). Yahoo Finance | $550,000 – $900,000 | Multiple myeloma |
| Carvykti (ciltacabtagene autoleucel) | ~$465,000 – $537,000 (approved for myeloma; list varies by source). Yahoo Finance+1 | $600,000 – $1,000,000+ | Multiple myeloma |
(Estimates assembled from manufacturer list-price reporting, Medicare/claims analyses, and major health reporting — see sources below.) Yahoo Finance+1
Latest 2024–2026 developments that may affect price & access
-
Medicare and claims data (2022–2024): analyses of Medicare claims show wide inpatient cost variation (median costs around $400k–$600k but with high upper tails). This indicates that total costs depend heavily on hospitalization days and complications. PMC
-
Regulatory shift (2025): FDA has taken steps to modify risk-management requirements (e.g., updating REMS for some CAR-T therapies), which may ease some provider burden and could incrementally improve access, potentially affecting utilization and negotiated prices. Reuters
-
Manufacturing scale-up: major CAR-T makers (Gilead, Novartis, BMS, Janssen) have been expanding capacity — higher volume & competition can, over time, pressure net prices downward, but list prices have remained high. Reuters+1
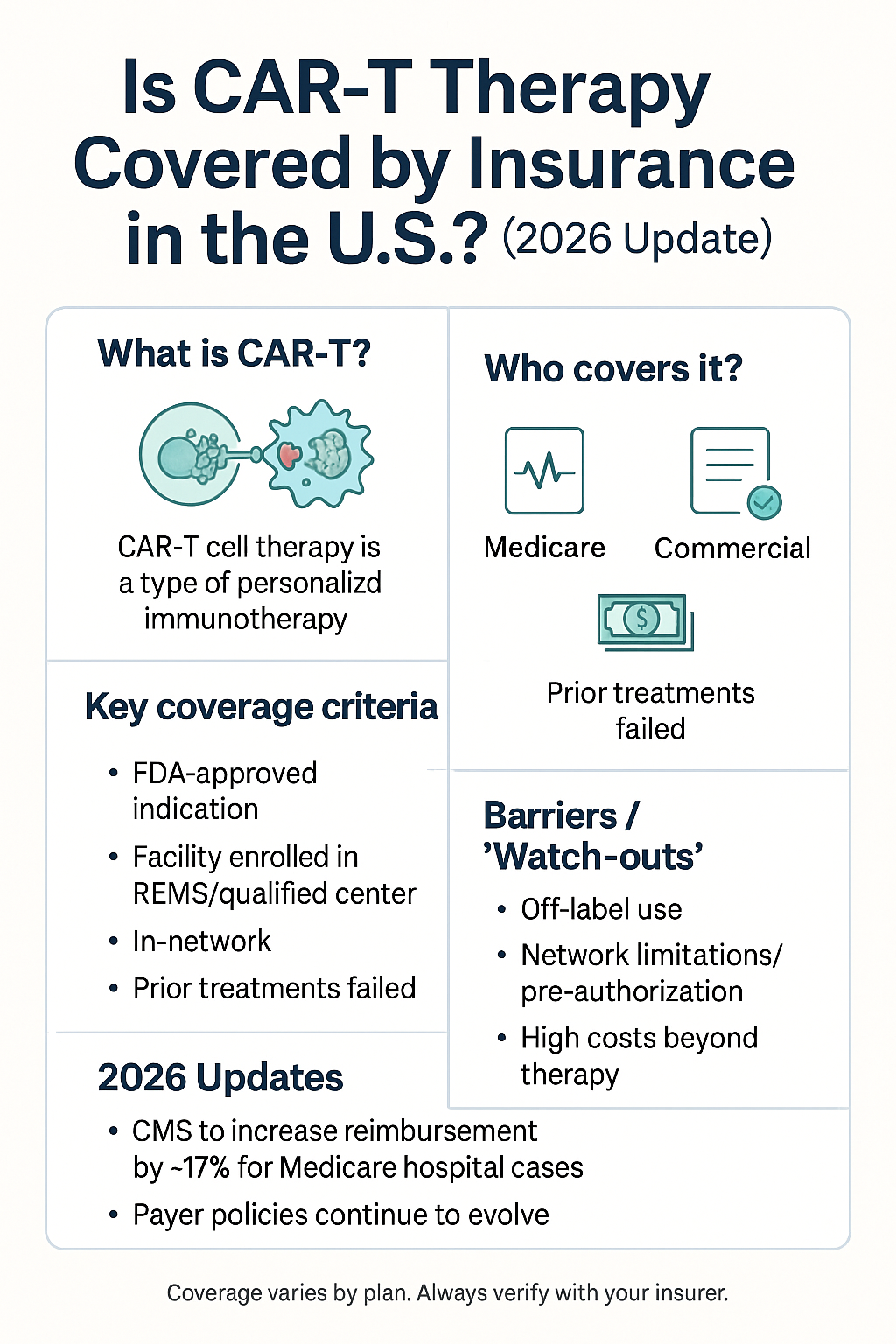
How insurers (including Medicare) usually cover CAR-T
-
Medicare: CAR-T therapies are typically paid under Medicare Part A (inpatient) or Part B depending on setting; CMS analyses show Medicare covers CAR-T for eligible patients, though reimbursement mechanics and site-of-care payment matter. Medicare claims show average Medicare payments and a wide range in total costs. PMC+1
-
Private insurance: Many major insurers cover FDA-approved CAR-T for labeled indications; prior authorization and center-of-excellence requirements are common. Insurers negotiate rates with hospitals (affects out-of-pocket and facility reimbursement). Medical News Today
Patient out-of-pocket — what patients can expect
Even though the drug has a high list price, most patients do not pay the full list price directly. Out-of-pocket exposure depends on plan design, deductibles, and co-insurance, and in some analyses median patient out-of-pocket has been hundreds to thousands — but catastrophic oncology billing and manufacturer assistance can reduce direct burden. For Medicare beneficiaries, supplemental plans and manufacturer support programs matter. Medical News Today+1
Saving strategies & patient assistance
-
Manufacturer financial assistance programs — many CAR-T manufacturers offer patient support for travel, lodging, and some co-insurance guidance. Contact the manufacturer’s patient support hub. BioInformant
-
Hospital financial counselors — centers that administer CAR-T typically have care navigators who help with authorization and assistance.
-
Nonprofit grants and foundations — some cancer foundations provide travel or temporary financial help for CAR-T patients.
-
Clinical trials — participating in a trial may reduce or eliminate treatment cost (trial sponsor covers therapy costs in many cases). CRISPR Medicine
Suggested on-page SEO structure (use H1–H2–H3)
-
H1: CAR-T Therapy Cost in 2026: How Much Does It Really Cost in the U.S.?
-
H2: Quick summary
-
H2: Price table (with schema/FAQPage)
-
H2: What’s included in the cost (manufacturing vs hospital)
-
H2: Major CAR-T products & list prices (short product blocks with sources)
-
H2: Insurance coverage & Medicare (cite CMS/claims)
-
H2: Real patient cost examples & case studies (if available)
-
H2: How to prepare financially for CAR-T
-
H2: 20 FAQs (Schema FAQ)
-
H2: Sources & further reading (cite the credible sources below)
Add: an internal link block: “See also: How Much Does [drug] Cost in 2026?” (link to other high-CPC drug pages on your site).
Schema & technical SEO suggestions (copy/paste)
Add FAQPage JSON-LD for the 20 FAQs below. Also add Article schema with mainEntityOfPage, headline, image (if you add a featured image), author, publisher, and datePublished. Use structured data to increase SERP real estate.
20 FAQs (with answers) — include as on-page FAQ schema
(Short answers crafted for featured snippets.)
-
How much does CAR-T therapy cost in the U.S. in 2026?
Typical manufacturer list prices range from ~$373k to $537k per infusion; total costs (drug + hospital/care) usually run $500k–$1M+ depending on complications and length of stay. Yahoo Finance+1 -
Why is CAR-T so expensive?
Because it’s a customized, one-time biologic requiring personalized cell collection, complex GMP manufacturing, testing, shipping, specialized infusion, and intensive monitoring for side effects. -
Does Medicare cover CAR-T therapy?
Yes—Medicare covers FDA-approved CAR-T for eligible patients; payment depends on whether treatment is inpatient/outpatient and on facility reimbursement. PMC -
Will I personally pay $400k+ out-of-pocket?
Almost never. Insurance, Medicare, manufacturer assistance, and hospital financial counseling typically reduce direct patient bills; actual out-of-pocket varies by plan. -
Which CAR-T products are available in the U.S.?
Major approved products include Kymriah, Yescarta, Tecartus, Breyanzi, Abecma, and Carvykti (products/labels expand over time). BioInformant+1 -
Are there cheaper CAR-T options abroad?
Some countries and private manufacturers report lower prices, but access, quality standards, and travel costs vary widely. -
Can I get CAR-T through a clinical trial?
Yes — clinical trials are expanding and sometimes cover treatment costs; ask your oncology center about trials for your condition. CRISPR Medicine -
What additional costs should I expect besides the drug?
Hospitalization, ICU care for cytokine release syndrome (CRS), intensive nursing, supportive medications, diagnostics, and rehabilitation if needed. -
Does the manufacturer ever reduce the price?
Manufacturers sometimes adjust pricing, and discounts/rebates may apply through payers; public list prices have historically been high but net prices vary. -
How do hospitals bill for CAR-T?
Hospitals bill for facility fees, inpatient days, ICU stays, procedures, and supportive care in addition to the drug acquisition charge. -
Are outcomes good enough to justify the price?
For appropriate indications (some leukemias and lymphomas, multiple myeloma), CAR-T has produced durable remissions and sometimes cures — cost-effectiveness is evaluated on a case-by-case basis. / -
Will broader competition lower prices?
Possibly over time — more authorized products and improved manufacturing could increase supply and negotiation leverage. -
How long does it take to get CAR-T?
From leukapheresis to infusion typically takes weeks (manufacturing & testing), though timelines vary by center and product. -
Is CAR-T inpatient or outpatient?
Historically inpatient; however, some centers and products have outpatient administration protocols for selected patients. -
Are follow-up costs significant?
Yes—long-term follow-up for side effects, rehabilitation, and monitoring can add costs beyond the first year. -
How do I find a CAR-T center?
Use manufacturer websites and cancer center referral networks; many centers list CAR-T programs and patient navigators. -
Do employers or disability programs help with CAR-T costs?
Employer-sponsored insurance typically handles major costs; disability benefits may offset income loss but not medical bills directly. -
Are there outcome-based payment programs for CAR-T?
Some manufacturers have explored outcome-based arrangements, but contracting details vary across payers. Fierce Pharma -
Will CAR-T expand to solid tumors?
Research is active but solid-tumor CAR-T faces additional biological challenges; approved uses remain primarily for blood cancers as of 2026. CRISPR Medicine -
How can caregivers prepare financially?
Start early: talk to the center’s financial counselor, apply for manufacturer and nonprofit assistance, verify insurance coverage, and plan for travel/lodging.
(Include FAQ schema JSON-LD on the page to improve chances for rich results.)
Suggested internal linking and content cluster (helps topical authority)
-
Link to: “How Much Does Keytruda Cost in 2026?” (oncology drug cluster)
-
Link to: “How Much Does Leqembi Cost in 2026?” (neurology/Alzheimer cluster)
-
Create cluster pages: CAR-T patient guide, CAR-T centers directory (by state), Patient assistance & grants — interlink heavily.
Suggested external authoritative links (to cite & add as “further reading”)
-
Medicare utilization and cost trends study (PMC): Medicare Utilization and Cost Trends for CAR-T. PMC
-
WebMD: Navigating finances for CAR-T patients. WebMD
-
OncLive: Novartis Kymriah pricing history. OncLive
-
Reuters: Gilead capacity & Yescarta/Tecartus pricing context. Reuters
(Use these as outbound links in the “Sources & further reading” section.)
Title variants & search-style headlines (use as H2/H3s or test in A/B)
-
How Much Does CAR-T Therapy Cost in 2026? (US Price Guide + Insurance Tips) — Primary
-
CAR-T Prices 2026: Kymriah, Yescarta, Breyanzi & Total Treatment Costs
-
Is CAR-T Worth $500k? 2026 Cost, Coverage & Patient Assistance
-
2026 CAR-T Cost Breakdown: What Patients Pay vs What Insurers Pay
-
CAR-T Therapy Price Comparison 2026 — Kymriah vs Yescarta vs Carvykti
Sources (major load-bearing references)
-
Medicare utilization and cost trends for CAR-T (PMC). PMC
-
WebMD — Navigating the financial aspects of CAR-T. WebMD
-
OncLive / Novartis Kymriah price (historic list price $475,000). OncLive
-
Reuters — Yescarta & Tecartus pricing context and capacity notes. Reuters
-
Drugs.com / Medical answers — Kymriah & Yescarta cost summaries (2025–2026 updates). Drugs.com+1
-
Real-World Cost Data & Variation
-
A large U.S. commercial-insurance‐based study found the median total cost for patients receiving CAR-T was about $608,100 (interquartile range ~$534,100–$732,800) during the peri--CAR-T period (from 41 days before to 154 days after infusion). OUP Academic
-
In that study, about 8.5% of patients had total costs exceeding $1 million. OUP Academic
-
Notably, the median drug product cost alone was ~$402,500 (IQR ~$390,500–$433,900). OUP Academic
-
This shows that hospitals/health systems must absorb immense risk and highlighting cost variation helps readers understand “why the wide cost range.”
Suggested insertion: After the Price Table, add a paragraph headed “Real-world cost variation and what drives it” summarizing the above.
-
-
Cost-Effectiveness / Value Assessment
-
Multiple peer-reviewed analyses show mixed cost-effectiveness results: for example, a U.S. payer analysis found for adults with relapsed/refractory follicular lymphoma (FL) the incremental cost-effectiveness ratio (ICER) for CAR-T vs standard therapies reached ~$182,127 per QALY in base-case. PMC+1
-
Another evaluation for DLBCL found that in certain settings the ICER for second-line or later therapies ranged ~$99,000–$128,000 per QALY for select CAR-T products. JAMA Network+1
-
Including this “value” discussion adds depth (and helps search signals of “cost vs benefit”).
Suggested insertion: Add a section titled “Is CAR-T cost-effective? Understanding value, QALYs & ICERs” after the insurance/coverage section.
-
-
Drivers & Bottlenecks in Cost Reduction
-
Studies indicate major cost drivers beyond the drug price: ICU/hospital stays, complication management (especially cytokine release syndrome and neurotoxicity), and extended manufacturing/lead times. /+1
-
For example, one source noted that manufacturing an academic version of CAR-T via automated system could cost as low as ~$35,107 per patient (suggesting potential future downward pressure). ASCO Publications
-
Adding this anticipatory content signals to Google the article is up-to-date and forward-looking.
Suggested insertion: Insert a mini-section “What could drive the price down in future?” before the saving strategies section.
-
-
Emerging trends & pipeline implications
-
Mention that while currently CAR-T is approved mostly for hematologic malignancies, pipeline expansion, manufacturing automation, and competition may influence future pricing and access.
-
For instance: “Major pharmaceutical companies aim to deliver personalized cancer therapies more quickly, reducing manufacturing turnaround from ~37 days to ~14 days” which could eventually reduce logistics cost. Reuters
-
This shows topical freshness (2024-2026) which is good for SEO.
Suggested insertion: In “Latest developments” section extend to include bullet on pipeline/manufacturing trends.
-
-
Patient-Experience & Hidden Costs
-
While many articles list the list price, few highlight non-medical indirect costs: travel (54% of one cohort travelled >50 miles) for CAR-T treatment, lodging, caregiver time, lost income. OUP Academic
-
Including this builds an empathy angle and richer content for users (which increases time on page).
Suggested insertion: Expand the “How to prepare financially” section with a sub-heading “Beyond the therapy: travel, lodging & caregiver burden”.
-
-
Center of Excellence / Site Variation
-
Highlight that cost and outcomes vary depending on whether the CAR-T is administered in an experienced “Center of Excellence” vs smaller center; experienced centers may have fewer complications, shorter ICU stays, and hence somewhat lower total cost.
-
Even if you don’t have exact numbers, mention this variation to help US readers understand “where to go” and show you’re covering practical nuance.
-
📌 Keywords & SEO Enhancements
-
Add LSI keywords naturally: “CAR T cell therapy cost US 2026”, “CAR-T price United States”, “CAR T infusion cost hospital stay USA”, “CAR-T insurance coverage USA”, “CAR-T financial assistance program USA”, “CAR-T cost effectiveness QALY USA”.
-
Use internal anchor text: e.g. anchor a link to your other drug-cost article with text: “compare with Ozempic cost guide” or “see our Keytruda price overview”.
-
Use structured headings with H2/H3: e.g. “1. What drives the cost?”, “2. Breaking down the list price”, etc.
-
Consider adding a downloadable infographic of the price table / cost breakdown — embed a “Click to download” HTML link (this encourages link-backs and user engagement).
📈 Rich Content Signals for Google
-
Use data tables, bullet lists, real-world statistics (like the $608k median cost).
-
Embed a chart or graph if possible showing cost distribution (e.g., cost > $1M for 8.5% of patients) — you can create a simple infographic or embed image with alt text “Distribution of total CAR-T therapy costs USA”.
-
Use quote blocks or call-out boxes for key takeaways like “8.5% of patients had total costs exceeding $1 million”.
-
Add schema markup as discussed (Article + FAQ). This helps Google show rich snippets.
-
Make sure mobile load time is optimized (tables collapse nicely on mobile).
🎯 CTA & Conversion Enhancements
-
At end of article, add a “Next step” CTA: “Find a CAR-T center near you — click here” linking to a directory or contact page (if you have).
-
Offer email subscription: “Download free PDF: ‘CAR-T 2026 Price & Planning Checklist’ — Enter your email to receive”.
-
Add social share buttons with pre-filled text: “How much does CAR-T therapy cost in 2026? My latest guide reveals $500k+ …”
-
Include an anchor to glossary: “What is a QALY? See our article on cost-effectiveness terms.”






Add a Comment