A Haitian Family’s Journey: Getting Keytruda Treatment in the U.S.
Introduction
When Keytruda (pembrolizumab) became a breakthrough cancer treatment in the United States, it gave hope to thousands of families. For one Haitian family living in Florida, this life-changing drug meant both a new chance at survival and a battle with the high cost of modern medicine. Their experience highlights the challenges many immigrants face when trying to access advanced cancer care in the U.S.
Marie’s Story
Marie, a Haitian mother of two, was diagnosed with metastatic melanoma in late 2024. Her oncologist recommended Keytruda, an FDA-approved immunotherapy that helps the body’s immune system recognize and fight cancer cells.
For Marie’s family, the news was bittersweet. While the drug promised better outcomes, it also came with complex questions about insurance coverage, copayments, and financial eligibility. Like many Haitian immigrants, Marie’s family had to navigate U.S. healthcare systems that can be confusing and expensive.
What Is Keytruda?
Keytruda (pembrolizumab) is a type of immunotherapy called a PD-1 inhibitor, which works by blocking the PD-1 protein on immune cells, helping them attack cancer cells more effectively.
As of 2025, the FDA has approved Keytruda for over 20 different cancer types, including:
-
Melanoma
-
Lung cancer (NSCLC)
-
Head and neck cancer
-
Bladder cancer
-
Kidney cancer
-
Some breast and colorectal cancers
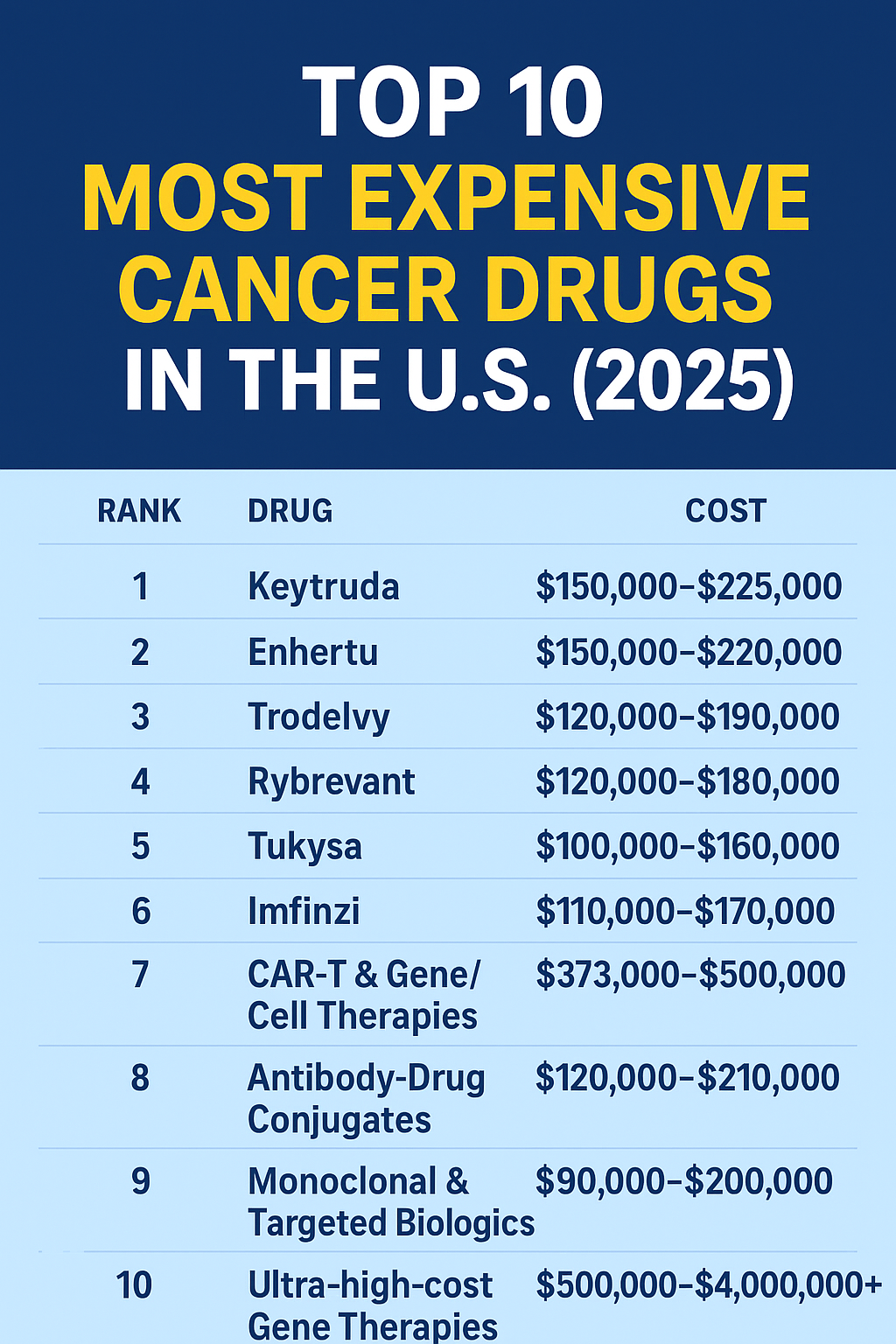
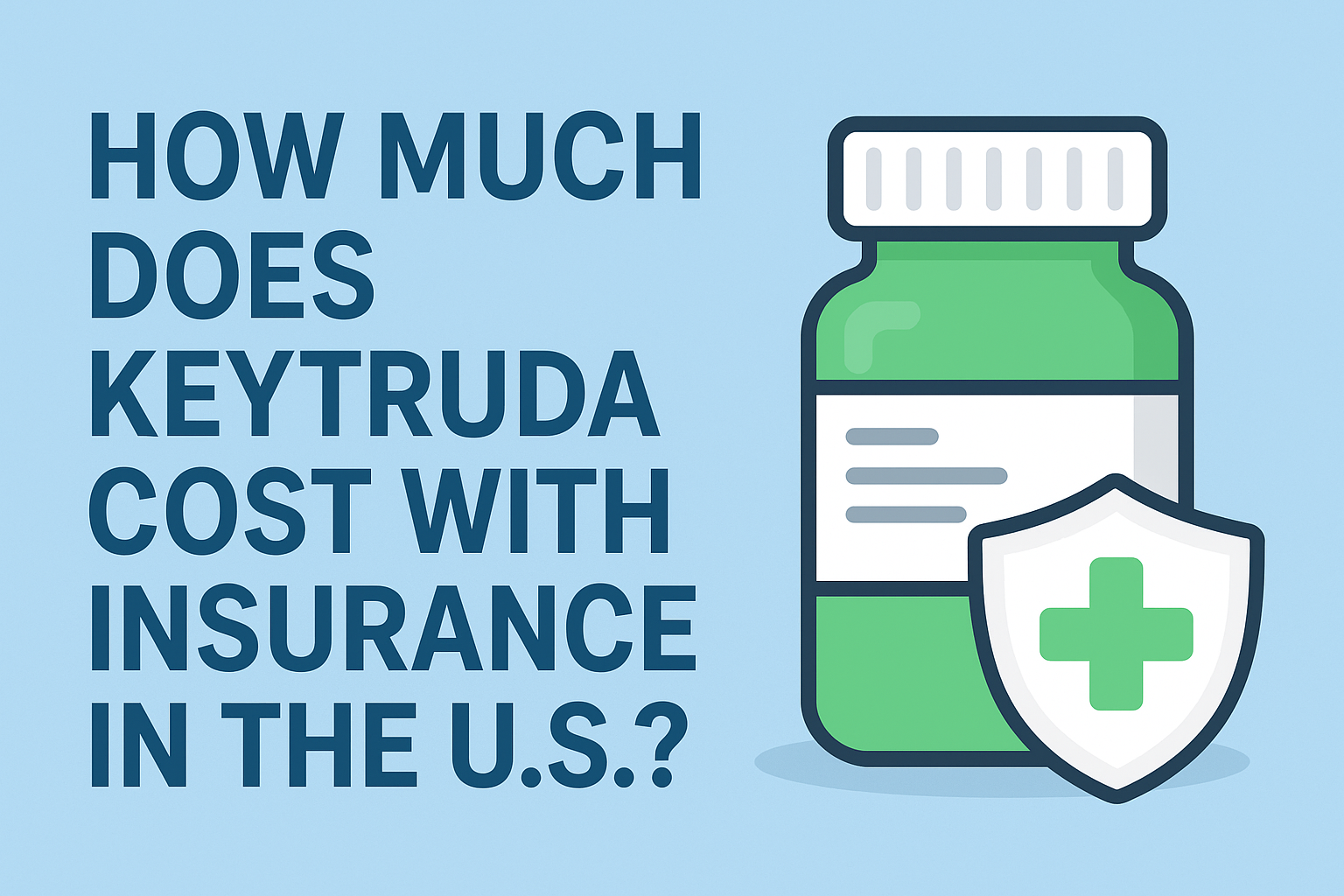
How Much Does Keytruda Cost in 2025?
According to the official manufacturer, Merck & Co., the list price for Keytruda is around $12,031 per 200 mg dose, usually given every three weeks. Patients who receive it every six weeks may pay around $24,062 per infusion before insurance.
(Source: keytruda.com, 2025 update)
Average U.S. Cost After Insurance
Most American patients with commercial insurance or Medicare Part B pay anywhere from $150 to $1,000 per infusion, depending on their deductible and coverage limits.
For Uninsured or Immigrant Patients
For families like Marie’s, who may not have full insurance, the total treatment cost can exceed $150,000 per year. However, Merck offers several Patient Assistance Programs (PAPs) and copay relief for eligible U.S. residents regardless of immigration status.
-
Merck Access Program: 1-855-257-3932
-
CancerCare Co-Pay Assistance: cancercare.org
-
PAN Foundation: panfoundation.org
These programs may cover all or part of the medication cost for low-income or uninsured patients.
The Role of the FDA and Patient Access
The U.S. Food and Drug Administration (FDA) continues to expand Keytruda’s approvals for new cancer types, including subcutaneous (under-the-skin) injection forms expected to simplify hospital visits in 2026. This innovation could benefit patients who live far from cancer centers or rely on public transport—an issue that affects many immigrant and minority families.
How Haitian and Caribbean Communities Can Get Help
Accessing advanced cancer drugs in the U.S. can be overwhelming, especially for Haitian families with limited insurance literacy or language barriers.
Here are practical steps to get support:
-
Ask your oncologist to connect you with a hospital financial navigator.
-
Apply directly through Merck’s Access Program—they can assist even if your immigration status is pending.
-
Contact Haitian community health centers in Florida, New York, or Massachusetts that partner with U.S. nonprofits.
-
Use Medicare’s “Extra Help” or Medicaid (if eligible) to reduce copays.
Conclusion
Marie’s journey with Keytruda shows both the promise and the price of innovation in modern cancer care. For Haitian and Caribbean families in the U.S., access to lifesaving drugs shouldn’t depend on income or paperwork. With the right support and awareness, more patients can receive treatments that truly extend life.






Add a Comment