How Much Does Keytruda (pembrolizumab) Cost in the U.S. in 2025? Prices, Insurance & Savings Options
Overview
Keytruda (pembrolizumab) is one of the most advanced immunotherapy drugs used to treat several types of cancer, including lung cancer, melanoma, and bladder cancer. While it’s a breakthrough in cancer treatment, the cost of Keytruda in the U.S. can be extremely high, especially for patients without insurance.
This guide breaks down current 2025 prices, insurance coverage, and available financial assistance programs.
💰 Keytruda Price in 2025 (U.S. Market)
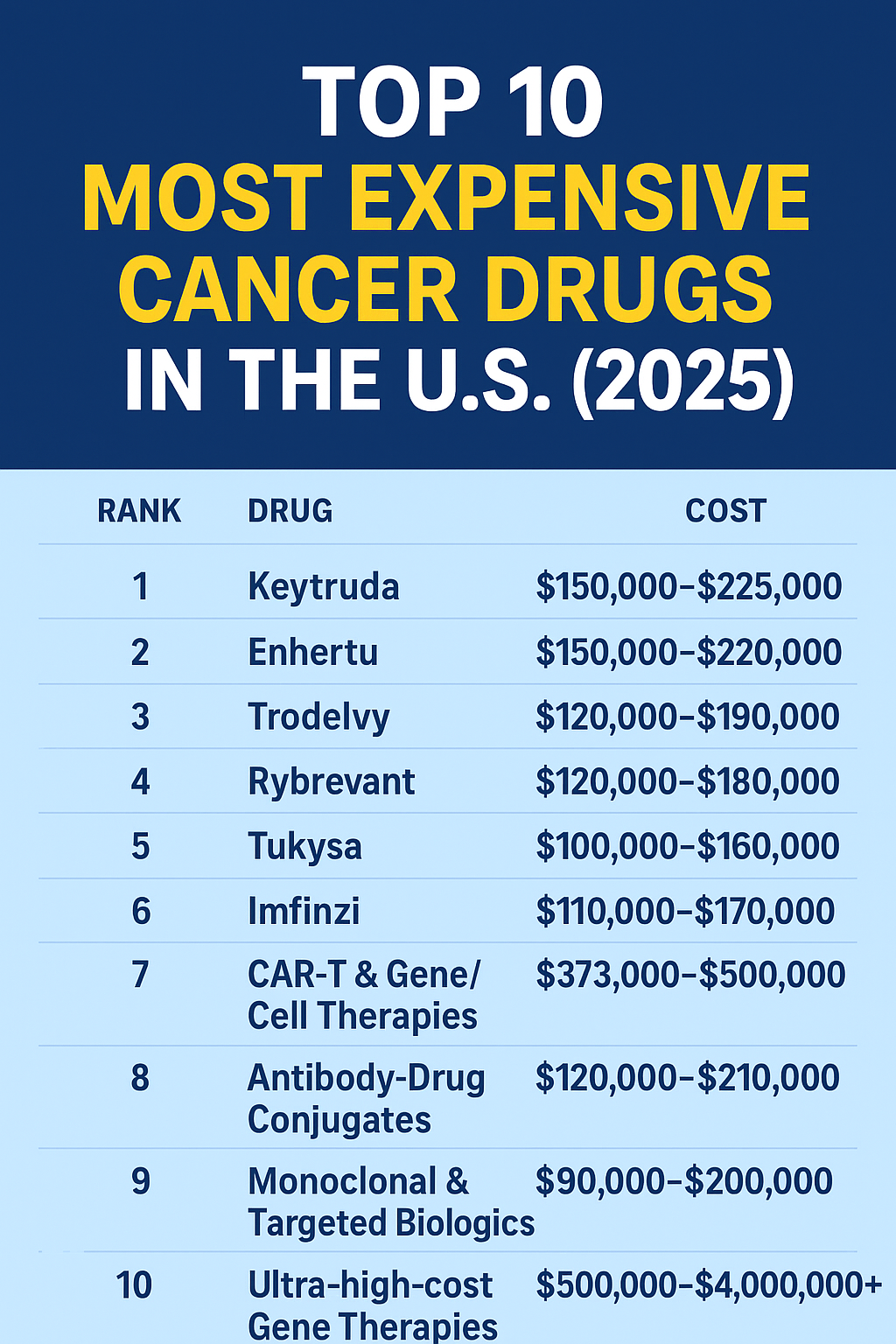
As of early 2025, the average cost of Keytruda is between $10,500 and $12,500 per dose (200 mg vial).
-
Per infusion: around $11,000
-
Monthly cost: about $22,000 (two doses per month)
-
Annual treatment: can exceed $150,000 – $200,000 per year
💡 These prices vary depending on hospital, pharmacy markup, and insurance plan.
🧾 Keytruda Cost with Insurance
If you have U.S. health insurance, your out-of-pocket cost can drop significantly:
-
Private insurance: usually covers 60%–80% of the total cost.
-
Medicare Part B: covers 80%, but the remaining 20% can still exceed $2,000 per infusion.
-
Medicaid: may cover most of the cost depending on your state.
👉 Tip: Always confirm coverage with your insurer before starting Keytruda treatment.
🚫 Keytruda Cost Without Insurance
For uninsured patients, the cost can be overwhelming — typically $10,000–$12,000 per dose, with total annual treatment costs easily exceeding $180,000.
However, Merck (the manufacturer of Keytruda) offers several assistance programs that can reduce or even eliminate the cost for eligible patients.
💡 Patient Assistance & Savings Programs
-
Merck Patient Assistance Program (PAP) – Offers free Keytruda to qualifying U.S. patients with limited income.
🔗 Visit: www.merckhelps.com -
Co-pay assistance – For insured patients with high deductibles.
-
Nonprofit foundations – Like CancerCare or HealthWell Foundation, which provide grants for cancer drug costs.
⚖️ Keytruda vs. Other Immunotherapy Drugs (Cost Comparison)
| Drug | Manufacturer | Average Cost per Dose | Coverage |
|---|---|---|---|
| Keytruda (pembrolizumab) | Merck | $10,500–$12,500 | 60–80% |
| Opdivo (nivolumab) | Bristol Myers Squibb | $9,800–$11,200 | 60–80% |
| Tecentriq (atezolizumab) | Genentech | $10,000–$12,000 | 60–80% |
Keytruda remains one of the most expensive but effective options for advanced-stage cancers.
📅 Will Keytruda Get Cheaper in 2026?
Experts predict slight price adjustments in 2026 due to insurance negotiations and new biosimilars under development.
However, major price drops are unlikely until after 2028 when patent protection may expire.
❓Frequently Asked Questions (FAQ)
Q1: How much does Keytruda cost per month with insurance?
With good insurance, most patients pay $1,500–$3,000 per month after deductibles and copays.
Q2: Is Keytruda covered by Medicare?
Yes, Medicare Part B covers Keytruda for approved cancer treatments.
Q3: Are there cheaper alternatives to Keytruda?
Drugs like Opdivo or Tecentriq can sometimes be cheaper, but suitability depends on cancer type and doctor recommendation.
Q4: Can I get Keytruda for free?
Yes — through the Merck Patient Assistance Program if you meet the financial eligibility criteria.
How Much Does Keytruda (Pembrolizumab) Cost in the U.S. in 2025? Prices, Insurance & Savings
Latest Price Data & Trends (2025)
Below is a table summarizing key published cost figures for Keytruda (pembrolizumab) in the U.S., plus comparisons and caveats:
| Scenario / Dosing Interval | Published Price (List / Reference) | Notes & Caveats |
|---|---|---|
| Single 200 mg dose (no insurance) | $11,337.36 | According to Medicare sources & manufacturer data Healthline |
| Every 3 weeks (list / manufacturer) | $11,564.16 | As per Keytruda listing for 3-week dosing Medical News Today+1 |
| Every 6 weeks (double dose equivalent) | $23,138.32 | Manufacturer list price when given every 6 weeks Medical News Today+1 |
| 6-week cost (GoodRx estimate) | $22,674.72 | GoodRx estimate combining two doses over 6 weeks GoodRx |
| 1-year annualized cost (typical dosing) | ~ $191,000 | Based on GoodRx projection GoodRx |
| Lower bound pharmacy estimate (intravenous solution) | $5,884.02 (4 mL vial) | From Drugs.com price guide Drugs.com |
| Flat–dose variation (site & insurer differences) | $12,000 – $30,000+ per infusion | Wide variation depending on provider, payer, location Serif Health |
Key insights:
-
The list (brand) prices are extremely high, but very few patients pay the full list cost.
-
Real “paid” rates vary dramatically depending on insurance contracts, hospital markups, location, and site-of-care. Serif Health+1
-
Because Keytruda is physician-administered, billing differences (hospital outpatient vs. infusion center vs. clinic) can produce large price spreads. Serif Health
-
Merck has acknowledged that Keytruda may be selected in 2026 for government price-setting (negotiated pricing) under U.S. drug pricing reform, which may constrain future price increases. Reuters+1
-
Analysts expect patent loss and biosimilar competition around 2028, which may push prices downward in later years. Fierce Pharma+1
Cost Differences: With Insurance vs. Without & What Patients Actually Pay
| Insurance / Coverage Type | Typical Patient Out-of-Pocket | Notes |
|---|---|---|
| Medicare Part B | 20% coinsurance + Part B deductible (~$240 in 2024) | After deductible, patient pays 20% of the Medicare-approved amount Healthline+1 |
| Medicare Advantage | Varies (some pay $0 to $925 per dose) | Some plans waive costs, others cap coinsurance Healthline |
| Private Insurance + Copay Assistance | $25 per infusion (for eligible patients) | Via Merck’s Co-pay Assistance Program, up to $25,000 annual cap merckaccessprogram-keytruda.com |
| No insurance / Uninsured | Essentially full list price (e.g. $11,000+ per dose) | Unless patient qualifies for assistance programs Healthline+2Drugs.com+2 |
| Pharmacy / discount programs | $5,800–$12,000 (for some vial pricing) | E.g. Drugs.com estimates for IV solution vials Drugs.com |
Note: Because Keytruda is infused at healthcare facilities, additional costs like infusion fees, facility fees, and administration costs are often billed separately and can add several thousand dollars per session.
20 Popular FAQs (U.S. Focus) + Answers
-
Q: How much does Keytruda cost per infusion (200 mg) in the U.S.?
A: As of recent data, the list price is about $11,337.36 per 200 mg dose without insurance. Healthline -
Q: What is the cost of Keytruda every 3 weeks?
A: The manufacturer lists $11,564.16 per dose on a 3-week schedule. Medical News Today+1 -
Q: What is the cost for dosing every 6 weeks?
A: Equivalent list cost is $23,138.32 for the 6-week period. Medical News Today+1 -
Q: How much is Keytruda per year (typical therapy)?
A: It can reach $190,000–$200,000+ annually under standard dosing. GoodRx+1 -
Q: Does Medicare cover Keytruda?
A: Yes — Keytruda infusions are generally covered under Medicare Part B as outpatient infusions, though patient pays coinsurance and deductible. Healthline+2Medical News Today+2 -
Q: What is the Part B deductible + coinsurance for Keytruda?
A: In 2024, the deductible is ~$240. After that, patients pay 20% of the Medicare-approved cost. Healthline -
Q: What do Medicare Advantage beneficiaries pay for Keytruda?
A: Out-of-pocket varies. Some pay $0, others up to $925 per infusion depending on plan. Healthline -
Q: Can private insurance cover Keytruda?
A: Yes — many private plans cover it under their medical benefit, but require prior authorization and may involve copays or coinsurance. Healthline+1 -
Q: Are there financial assistance programs for Keytruda?
A: Yes — e.g. Merck Co-pay Assistance Program (for insured) and Merck Patient Assistance Program (for uninsured/low income) merckaccessprogram-keytruda.com+2Keytruda+2 -
Q: How much can co-pay assistance reduce cost?
A: Eligible privately insured patients may pay just $25 per infusion, up to a $25,000 annual cap. merckaccessprogram-keytruda.com -
Q: Can uninsured patients receive Keytruda for free?
A: If they qualify for the Merck Patient Assistance Program, they may receive it at no cost. merckaccessprogram-keytruda.com+2Keytruda+2 -
Q: Why is Keytruda so expensive compared to other drugs?
A: Because it’s a biologic, with costly research, development, manufacturing, and exclusivity rights. Healthline+2Medical News Today+2 -
Q: Are there biosimilars for pembrolizumab (Keytruda)?
A: Not currently approved; Keytruda is still a brand biologic. Medical News Today+1 -
Q: How does site of care affect pricing?
A: Infusions delivered in hospital outpatient settings often cost more than in outpatient clinics due to facility fees. Serif Health -
Q: What is the variation in reimbursement among payers?
A: Commercial reimbursements can range from under $12K to over $30K per infusion depending on provider and payer contracts. Serif Health -
Q: Will Keytruda’s price drop in the future?
A: Possible — Keytruda is expected to become eligible for government price setting by 2026, with effects starting in 2028. Reuters+2Fierce Pharma+2 -
Q: What is the patent expiration timeline?
A: Patent exclusivity is expected to expire around 2028, enabling biosimilar competition. Fierce Pharma+1 -
Q: Is Keytruda cost-effective at current prices?
A: Some health economics models suggest it is not cost-effective at current pricing (e.g. cervical cancer) unless price or duration is reduced. JAMA Network+2Applied Clinical Trials Online+2 -
Q: How much would Keytruda need to drop to be cost-effective?
A: In one analysis, monthly price would have to fall from $16,990 to ~$9,190 (−45.6%) or max duration reduced. JAMA Network+1 -
Q: What additional costs should patients expect (beyond drug cost)?
A: Infusion administration fees, facility fees, lab tests, imaging, supportive medications, and hospital facility overhead.
High-CPC Keywords to Target
These are high-value keyword ideas (especially in U.S. medical / pharma niches) that you should integrate:
-
keytruda cost
-
keytruda price per infusion
-
pembrolizumab cost
-
keytruda cost with insurance
-
keytruda cost without insurance
-
keytruda patient assistance
-
pembrolizumab price in USA
-
how much does keytruda cost
-
keytruda copay assistance
-
keytruda co pay program
-
keytruda annual cost
-
keytruda Medicare cost
-
keytruda insurance coverage
-
keytruda infusion cost
-
keytruda cost 2025
-
keytruda vs opdivo cost
-
cost of immunotherapy cancer
-
cost of pembrolizumab 200 mg
-
pembrolizumab cost comparison
-
cost of checkpoint inhibitors
🆕 Latest Developments & Forecasts (as of 23-Oct-2025 & looking toward 2026)
-
Price increases: ~2% for Keytruda injection
Recent pricing reports show that the list price of Keytruda injection was raised by about 2% in 2025. 46brooklyn Research
This small percentage bump is consistent with typical annual price inflation for high-cost biologics, but keep in mind this is the list (sticker) price, which many payers don’t actually pay due to rebates, discounts, and negotiated contracts. 46brooklyn Research -
Qlex (subcutaneous version) priced at parity with IV Keytruda
Merck has introduced or is pushing a subcutaneous formulation known as Keytruda Qlex (or sometimes "Qlex version") and has reportedly priced it at parity with the traditional intravenous version (i.e., ~$12,000 for a 3-week equivalent dose) BioPharma Dive+2PharmaVoice+2
A key rationale is that many payers and providers may prefer the subcutaneous (SC) route because it is faster, more convenient, and may reduce facility/infusion chair costs (less time, fewer resources) PharmaVoice+2BioPharma Dive+2 -
Government price setting & selection delays
-
Merck expects that Keytruda will be chosen for U.S. government price negotiation in 2026, which would then cause negotiated pricing to take effect starting January 1, 2028. Seeking Alpha+3Reuters+3Pearce IP+3
-
However, recent legislative changes have delayed Keytruda’s eligibility under the Inflation Reduction Act’s drug price negotiation rules from 2026 to 2027, meaning the negotiated price would take effect in 2029 if selected. KFF
-
This delay arises because of rules about how long a drug held an orphan designation before broader approval counts toward the “11-year” window. KFF
-
-
Sales & market risks due to patent expiry & competitive pressures
-
Keytruda accounted for nearly 46% of Merck’s total sales in 2024, making it extremely critical to Merck’s revenue. Fierce Pharma+3Reuters+3Seeking Alpha+3
-
Analysts estimate Keytruda’s U.S. sales could peak near $36 billion by 2028 but may decline significantly in subsequent years—perhaps falling to $15–20 billion—once patent protection ends and biosimilars enter the market. Nasdaq
-
The patent and exclusivity protections are expected to expire in 2028, enabling biosimilar competition. Seeking Alpha+3Fierce Pharma+3Reuters+3
-
Because of the looming patent cliff, Merck is pushing "next-generation" versions (like Qlex) and pipeline products to preserve market share. Pearce IP+3Seeking Alpha+3Yahoo Finance+3
-
-
Cost-effectiveness scrutiny and pressure to reduce price
-
A recent cost-effectiveness analysis of Keytruda (from the KEYNOTE-A18 trial) concluded that unless the monthly price dropped from $16,990 to about $9,190 or treatment durations were limited, Keytruda would not be considered cost-effective under common U.S. willingness-to-pay thresholds. Applied Clinical Trials Online
-
This kind of analysis adds public and payer pressure for price moderation or limits on indications. Applied Clinical Trials Online
-
-
New FDA approvals / label expansions for pembrolizumab
-
In March 2025, the U.S. FDA granted full approval (beyond accelerated status) for pembrolizumab in combination with trastuzumab + chemotherapy for HER2-positive gastric / gastroesophageal junction (GEJ) adenocarcinoma with PD-L1 expression (CPS ≥ 1). Wikipedia
-
In June 2025, the FDA also approved pembrolizumab as neoadjuvant + adjuvant therapy in resectable locally advanced head and neck squamous cell carcinoma (for tumors expressing PD-L1) — the first perioperative approval in head & neck cancer. Wikipedia
-
📝 Suggested Insert (update snippet) for your article
You can add a small “Recent Updates (as of October 2025 / Outlook to 2026)” section in your article, for example:
Recent Updates & Projections (October 2025 – 2026)
• Keytruda list prices increased by ~2% in 2025 (pre-rebate).
• Merck has launched (or is pushing) a subcutaneous version (Keytruda Qlex), priced at parity with IV dosing, which may reduce facility/infusion costs and accelerate uptake.
• Under U.S. drug pricing reforms, Keytruda was expected to enter Medicare negotiation in 2026, but a legislative delay pushed its eligibility to 2027, making negotiated prices effective in 2029 if selected.
• Patent protection for Keytruda in the U.S. expires around 2028, ushering in biosimilar competition that could drive prices down significantly.
• Analysts forecast U.S. sales may peak near $36 billion by 2028, then decline sharply post-patent expiry.
• Cost-effectiveness analyses warn that current pricing is not sustainable unless prices drop or treatment durations are curtailed.
• Pembrolizumab (Keytruda) gained expanded FDA approvals in 2025, including for HER2-positive gastric cancer and perioperative head & neck cancer, which may broaden its usage (and pricing pressures).
📊 Projected Revenue & Market Trends for Keytruda / “Keytruda + Qlex” (2026–2028)
| Year | Revenue Estimate / Market Size | Key Drivers & Notes |
|---|---|---|
| 2025 (base year) | ~$31.5–32.7 billion (Keytruda + early Qlex) | According to Evaluate consensus, Keytruda sales are projected to peak near $32.7B in 2026 when combined with the new subcutaneous version. PharmaVoice |
| 2026 | ~$33.6 billion combined (≈ $32.7B Keytruda + $0.9B Qlex) | Qlex is expected to begin contributing meaningfully, capturing early market share. PharmaVoice |
| 2027 | ~$35.4 billion combined | Qlex adoption accelerates, softening the decline in overall Keytruda revenue. PharmaVoice |
| 2028 | ~$32.7 billion (Keytruda + Qlex) | As Keytruda’s exclusivity loss begins, Qlex helps cushion revenue drop. PharmaVoice+1 |
| Post-2028 (2030+) | Downtrend expected | With patent expiration and biosimilar entry, forecasts show a steep decline in revenue after 2028. PharmaVoice+1 |
Notes on the forecasts & assumptions:
-
The “combined” numbers include both the traditional intravenous Keytruda and the newer subcutaneous version (often called Qlex) as it gains approval and market adoption. PharmaVoice
-
Qlex is expected to be priced at parity with the IV version (i.e. not discounted), enhancing the likelihood that payers and providers accept it as a replacement. BioPharma Dive+1
-
Analysts expect Qlex to capture 30%–40% of the eligible patient market within 18–24 months of launch, especially because of its shorter administration time and likely lower infusion / facility costs. Investing.com+2BioPharma Dive+2
-
Some forecasts suggest Keytruda will remain a top revenue driver through 2028 before decline. Drug Discovery Trends+2PharmaVoice+2
-
As Keytruda loses patent protection in 2028, biosimilars are expected to enter, exerting downward pressure on pricing and margins. PharmaVoice+3Reuters+3MedPath+3
💵 Projected Cost / Price Pressure Scenarios (for Patients & Payers)
While there is no definitive “retail cost forecast” for patients (since pricing depends heavily on insurer negotiations, rebates, and regulatory constraints), some plausible scenarios to consider in your article:
-
After 2028, list prices might be pressured downward by 15%–30% or more in some settings, especially for biosimilar competition.
-
Some payers may push patients toward the subcutaneous version (Qlex) because of lower infusion costs and logistical convenience, which may reduce the “site-of-care markup” for patients.
-
Government-negotiated pricing (expected under U.S. law) may force reduction of prices for Medicare and other government payers; this might be partially passed onto patients in the form of lower out-of-pocket rates or capped co-insurance for select drugs. Reuters+1
-
Manufacturers often respond to looming patent cliffs by extending exclusivity via newer formulations (such as Qlex) so that list pricing power is preserved in part. BioPharma Dive+1
📌 Suggested Insert / Update Snippet for Your Article
You could add a short “Forecasts & Cost Scenarios (2026–2028)” section:
Forecasts & Cost Scenarios (2026–2028)
• Consensus analyst forecasts project Keytruda + Qlex revenue climbing to ~$33–35B in 2026–2027 before easing in 2028. PharmaVoice+2Grand View Research+2
• The subcutaneous formulation (Qlex) is expected to capture 30–40% of the patient market within 1–2 years of launch, particularly because of ease and likely lower facility costs. Investing.com+2PharmaVoice+2
• After 2028, biosimilar competition and loss of exclusivity may drive price reductions of 15–30% or more in many settings.
• Under U.S. drug-pricing reforms, Keytruda may be subject to government price negotiation, possibly reducing prices for Medicare and other payers starting in 2028. Reuters+2Lexology+2
• Patients may see lower out-of-pocket costs if payers shift more usage to Qlex or push for site-of-care cost reductions.
• Manufacturers often roll out patent-extending formulations to preserve pricing power, and Merck’s push of Qlex is one example. BioPharma Dive+2PharmaVoice+2
Keytruda Alternatives in 2026: Cheaper or Comparable Immunotherapy Options
As Keytruda (pembrolizumab) prices remain high in 2025–2026, many patients and doctors are exploring alternative FDA-approved immunotherapy drugs that may offer similar results at a lower cost. Below is a comparison of leading options available in the U.S. market.
1. Opdivo (nivolumab) – made by Bristol Myers Squibb
-
Average cost per dose: $9,800–$11,200
-
Type: PD-1 inhibitor (same class as Keytruda)
-
Main uses: Lung cancer, melanoma, kidney cancer, and colorectal cancer
-
More info: www.opdivo.com
2. Tecentriq (atezolizumab) – made by Genentech (Roche)
-
Average cost per dose: $10,000–$12,000
-
Type: PD-L1 inhibitor
-
Main uses: Lung, bladder, and triple-negative breast cancer
-
More info: www.tecentriq.com
3. Imfinzi (durvalumab) – made by AstraZeneca
-
Average cost per dose: $8,500–$10,500
-
Type: PD-L1 inhibitor
-
Main uses: Lung cancer and bladder cancer
-
More info: www.imfinzi.com
4. Libtayo (cemiplimab-rwlc) – made by Regeneron/Sanofi
-
Average cost per dose: $8,000–$10,000
-
Type: PD-1 inhibitor
-
Main uses: Skin cancer (CSCC), NSCLC, and cervical cancer
-
More info: www.libtaayo.com
5. Bavencio (avelumab) – made by Pfizer and Merck KGaA
-
Average cost per dose: $7,000–$9,000
-
Type: PD-L1 inhibitor
-
Main uses: Bladder cancer, Merkel cell carcinoma
-
More info: www.bavencio.com
Key Takeaways
-
Keytruda remains the benchmark for immunotherapy but is among the most expensive options.
-
Opdivo and Tecentriq are the most common alternatives in 2026, often with comparable efficacy and slightly lower prices.
-
Imfinzi and Libtayo provide effective options for lung and skin cancers, often at lower costs.
-
Bavencio offers one of the most affordable checkpoint inhibitor options for eligible patients.
For patients in the U.S., discussing these alternatives with your oncologist can help identify more cost-effective and insurance-friendly options — especially if Keytruda coverage or affordability is a challenge.




Add a Comment