Introduction
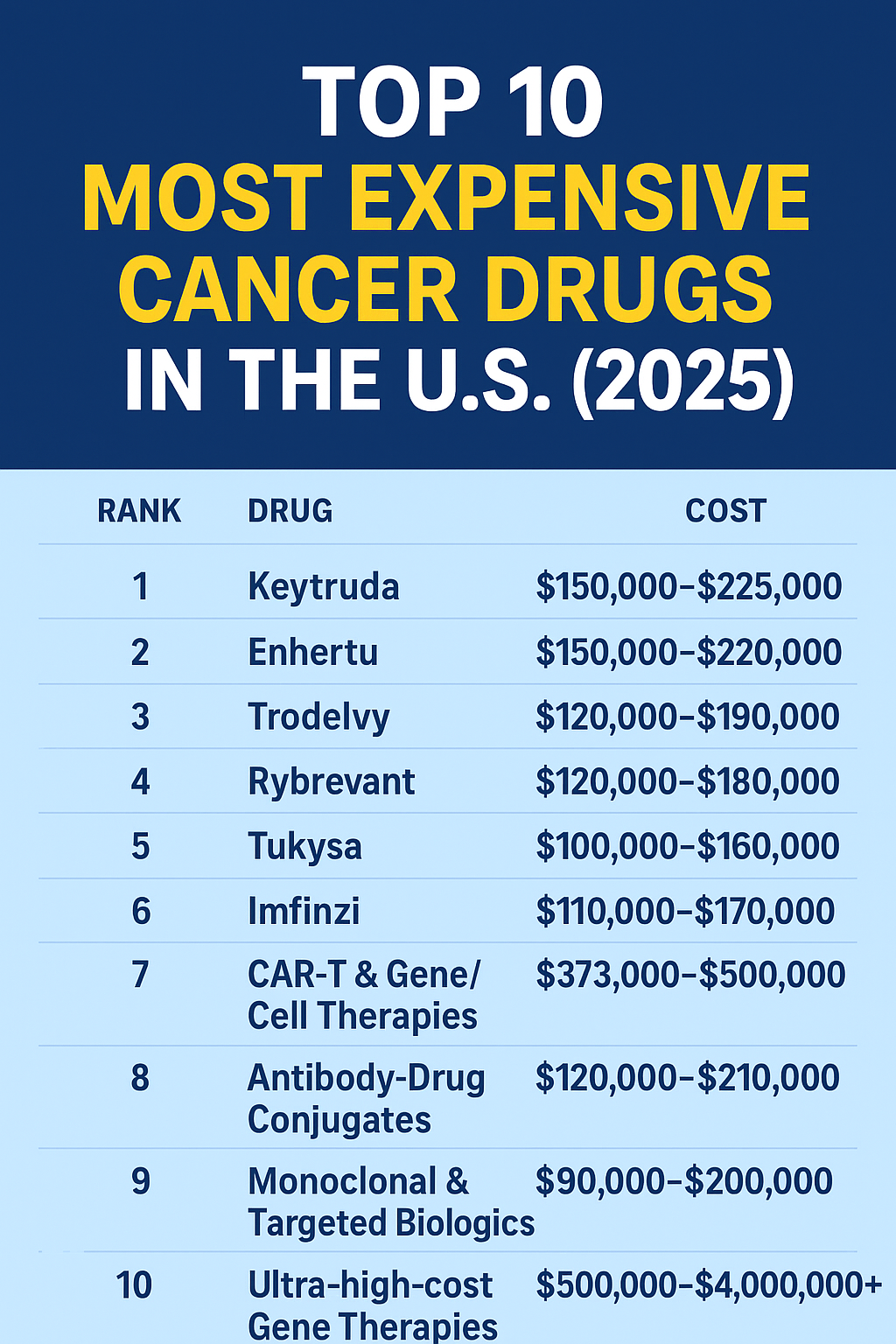
Keytruda (generic name pembrolizumab) is one of the leading cancer immunotherapy medications, used in multiple cancer types including non–small cell lung cancer, melanoma, head & neck cancers, and more. Because it is a biologic therapy, Keytruda comes with a very high cost, which can be a barrier for many patients.
In 2026, patients are facing new pressures: regulatory changes including Medicare drug price negotiation are on the horizon, and list prices may shift. In this article, we break down:
-
What the “list price” of Keytruda is in 2025–2026
-
What real patients typically pay (commercial insurance, Medicare, Medicaid, uninsured)
-
How insurance and government programs cover Keytruda
-
Financial assistance, copay support, and patient aid programs
-
Price comparisons, trends, and forecasts
-
Frequently asked questions
Our aim: equip you (patients, caregivers, providers) with clarity and up-to-date insight into what to expect in cost and support in 2026.
The List Price of Keytruda (Wholesale Acquisition Cost)
Before discounts, rebates, or insurance adjustments, pharmaceutical companies publish a “list price” (also called wholesale acquisition cost, WAC). These figures are mostly theoretical for most patients, but they give a baseline.
According to Keytruda’s official site (as of late 2025):
-
A 200 mg dose given every 3 weeks: ~ USD $12,031.36 keytruda.com
-
A 400 mg dose every 6 weeks: ~ USD $24,062.72 keytruda.com
Other sources estimate similar numbers (e.g. ~$11,337.36 for 200 mg) Healthline+2Healthline+2.
One “price transparency” analysis shows Medicare’s reimbursement basis (Average Sales Price) for pembrolizumab is $58.562 per mg (so ~ $11,712 for 200 mg) for the July–September 2025 quarter. serifhealth.com
Important caveat: Real patient out-of-pocket costs rarely match the list price, because of discounts, rebates, negotiation, insurance coverage, and assistance programs.
What Patients Actually Pay in 2026
Below is a synthesized view of what patients often pay in different insurance / coverage scenarios. These are estimates based on publicly available data as of 2025; actual 2026 numbers may vary slightly due to plan changes or policy shifts.
| Coverage Scenario | Estimated “List / Reference” Price* | Typical Patient Out-of-Pocket / Copay Range | Notes / Conditions |
|---|---|---|---|
| Commercial / Private Insurance | ~$12,000 per 200 mg dose (3-week schedule) | Often, $25 per infusion (if enrolled in copay assistance) merckaccessprogram-keytruda.com+3Drugs.com+3merckaccessprogram-keytruda.com+3 | Requires that the plan covers Keytruda under medical benefit. Many patients are eligible for Merck’s copay assistance. |
| Medicare Part B / Original Medicare | Reimbursement ~ $11,700 per dose (200 mg) under ASP methodology serifhealth.com+2medicalnewstoday.com+2 | After deductible & 20% coinsurance: ~$1,300 – $2,100 per infusion (typical) medicalnewstoday.com+1 | Medicare may require medical necessity. Some Medicare Advantage plans may cover more or have different cost‐sharing. |
| Medicare Advantage / Medicare Part C | — | Some patients pay $0 (no out-of-pocket) in certain plans; others pay up to ~$925 or more per infusion Healthline+1 | Cost sharing and coverage depend heavily on the specific plan’s formulary and benefit design. |
| Medicaid | — | Typically $4 to $8 per Keytruda infusion Drugs.com+1 | Varies by state. Medicaid covers most medically necessary cancer therapies, but rules differ by state. |
| Uninsured or underinsured | ~$12,000+ per dose | Could be extremely high unless assistance programs step in | Uninsured patients may receive Keytruda free or at a discount via Merck assistance, if eligible. merckaccessprogram-keytruda.com+3merckaccessprogram-keytruda.com+3merckhelps.com+3 |
* “Reference” price is the baseline list or ASP-based pricing; does not include infusion administration or hospital facility fees.
Notes & Observations
-
The patient’s actual cost depends on many factors: deductibles, coinsurance percentages, out-of-pocket maximums, whether Keytruda is billed under medical vs pharmacy benefit, whether prior authorization is required, and where infusion occurs.
-
Commercial insurers often negotiate significantly with providers, so the negotiated rate could be higher or lower than Medicare’s reference rate. One analysis found negotiated commercial rates for pembrolizumab vary by up to 5–10× across providers and regions. serifhealth.com
-
Many patients never pay the full 20% under Medicare because supplemental (Medigap) or secondary insurance may cover coinsurance.
-
Infusion center fees, facility fees, labs, imaging, and supportive care costs are not usually included in the tables above but can add substantially to the total cost of treatment.
Why Are the Prices So High?
Understanding the cost structure helps explain why patients often see such high prices for biologics:
-
Research & Development Costs
Developing immunotherapy drugs like Keytruda requires large investments in preclinical studies, multiple clinical trials, regulatory approvals, and post-market safety monitoring. -
Biologic & Manufacturing Complexity
Biologics (antibodies) are more complex to manufacture than small molecules, requiring specialized facilities, strict quality controls, and often more expensive input raw materials. -
Patent & Market Exclusivity
Keytruda has patent and exclusivity protections, which limit competition from biosimilars until expiration (expected around 2028) Reuters. -
Rebates & Discounts Hidden from Patients
The list price is often a starting point — manufacturers may provide rebates to pharmacy benefit managers (PBMs) or payers behind the scenes, which reduce net cost to payers but rarely lower the list price seen by patients. -
Price Negotiation Policies on the Horizon
Under the Inflation Reduction Act, certain high-cost drugs will become subject to Medicare negotiation starting in 2026 (with implementation in 2028) — Keytruda is expected to be among them. Reuters -
Geographic & Provider Markup Variability
Location, billing class (hospital outpatient vs infusion center), and local market competition influence how much providers charge beyond base drug costs.
Regulatory & Policy Developments in 2026
Medicare Drug Price Negotiation
Merck expects Keytruda to be selected for government negotiation under Medicare’s drug pricing reforms beginning in 2026, with new pricing effective January 1, 2028. Reuters This means that while prices in 2026–2027 may remain similar, the landscape is likely to shift. Price reductions of 38–79% have been seen in other negotiated drugs. Reuters
Patent Expiry & Biosimilars
Keytruda’s key patents are expected to expire or face challenges around 2028, opening the possibility of biosimilar competition which could drive price down. Reuters
Payer Pressure & Value-Based Deals
Payers (insurers) increasingly are pushing back on high-priced cancer therapies, seeking value-based agreements, outcomes-based pricing, or restricting use to patients most likely to benefit.
Transparency & Price Reporting
New regulations on price transparency may force providers and payers to disclose more about negotiated rates and patient cost shares, which could pressure price moderation.
Financial Assistance, Copay & Patient Support
Given the high cost, Merck and several nonprofit organizations offer financial support to eligible patients.
Merck / Manufacturer Support Programs
-
Merck Co-Pay Assistance Program for Keytruda
For patients with private insurance, this program helps reduce their out-of-pocket copay: eligible patients may pay as little as $25 per infusion. Maximum benefit through the program is $25,000 per eligibility period. merckaccessprogram-keytruda.com+2Drugs.com+2
This program is typically not available to patients on government programs (Medicare, Medicaid). merckaccessprogram-keytruda.com+1 -
Merck Patient Assistance Program (PAP)
For uninsured or underinsured patients who meet income criteria, Merck may provide Keytruda free of charge (or at reduced cost). NeedyMeds Blog+3merckhelps.com+3merckaccessprogram-keytruda.com+3
Income thresholds: e.g., individual ≤ ~$78,250, couple ≤ ~$105,750, family of 4 ≤ ~$160,750 (these numbers are representative; check current criteria). merckhelps.com
Nonprofit & Independent Foundations
-
Organizations like HealthWell Foundation, Patient Access Network (PAN), NeedyMeds, or independent copay assistance foundations may offer additional support. NeedyMeds Blog+2Healthline+2
-
Some states have cancer drug assistance programs or additional charity funds.
Tips to Maximize Assistance
-
Apply early (some programs require enrollment before or soon after first dose).
-
Ensure your oncologist or care provider helps submit required paperwork (financial declarations, proof of income, prescriptions).
-
Keep in mind, assistance programs are not insurance and may have annual caps.
-
Always verify whether your treatment center and insurance are eligible in the program’s terms.
Comparison: Keytruda vs Alternative Immunotherapies
To help patients and providers understand how Keytruda stacks up, here’s a simplified comparison (values approximate, illustrative):
| Drug / Comparator | Typical Dose / Schedule | Estimated Cost per Cycle* | Notes |
|---|---|---|---|
| Keytruda (pembrolizumab) | 200 mg every 3 weeks | ~$12,000+ | Most data used above |
| Nivolumab (Opdivo) | varies by indication | Similar high cost | Competes in many cancer types |
| Atezolizumab (Tecentriq) | varies | high biologic cost | another PD-L1 inhibitor |
| Durvalumab (Imfinzi), Avelumab, Cemiplimab | varies | high cost | used in specific cancers |
* These are drug costs only and do not include administration, facility fees, imaging, etc. Biologic immunotherapies are uniformly expensive, and coverage challenges are similar across them.
In many cases, comparative cost is not the key driver—efficacy, side effect profile, biomarker suitability, and insurance coverage often dominate choices.
Forecast & Trends for 2026
-
Moderate price increases: Manufacturers historically raise list prices annually (e.g., inflationary adjustments). Some small increases in 2026 are likely, unless constrained by policy.
-
Rebate & discount pressure: As Medicare negotiation looms, insurers may demand larger rebates or discounts in 2026 to prepare their cost exposure.
-
Increased cost sharing pushback: Patients and providers may resist high out-of-pocket burdens; insurers may adopt stricter utilization management (prior authorizations, step therapy).
-
Entry of biosimilars (post-2028): While not directly in 2026, expectation of biosimilar entry may influence contract negotiations and future premiums.
-
More transparency & legislative oversight: Greater regulatory scrutiny may limit aggressive markups or force disclosure of real net prices.
20 Popular FAQs (with Answers)
Below are 20 common questions U.S. patients often search for related to Keytruda cost and coverage, along with answers.
-
Q: How much does Keytruda cost per dose without insurance?
A: A 200 mg dose (every 3 weeks) is listed at ~ $11,300 to $12,000+; a 400 mg dose (every 6 weeks) around $24,000. Healthline+3Healthline+3keytruda.com+3 -
Q: Does Medicare cover Keytruda?
A: Yes. Medicare Part B typically covers outpatient infusions, subject to deductible and 20% coinsurance. medicalnewstoday.com+2Healthline+2 -
Q: How much do Medicare patients pay out of pocket?
A: After the Part B deductible, many patients pay ~ $1,300–$2,100 per infusion; some pay less if they have supplemental coverage. medicalnewstoday.com+1 -
Q: Is Keytruda covered under Medicare Advantage?
A: Yes, many Medicare Advantage plans include coverage. Some patients may pay $0 out-of-pocket, depending on plan benefits. Healthline+1 -
Q: Will Medicare negotiation reduce Keytruda’s cost in 2026?
A: Keytruda is anticipated to be in the pool of drugs eligible for Medicare negotiation. However, price reductions would take effect in 2028, not immediately in 2026. Reuters -
Q: How much do Medicaid patients pay for Keytruda?
A: Often $4 to $8 per infusion, though state rules vary. Drugs.com+1 -
Q: What if I don’t have insurance—can I still get Keytruda?
A: Possibly. If eligible, Merck’s Patient Assistance Program may provide Keytruda free or at reduced cost. NeedyMeds Blog+3merckhelps.com+3merckaccessprogram-keytruda.com+3 -
Q: How do I qualify for Merck’s copay assistance?
A: You must have private insurance that covers Keytruda (under medical or pharmacy benefit) and meet eligibility rules. The program is not valid for those on government programs. merckaccessprogram-keytruda.com+1 -
Q: Is there a maximum copay assistance benefit?
A: Yes — the maximum benefit is $25,000 per patient per eligibility period. merckaccessprogram-keytruda.com -
Q: Does Keytruda have a generic or biosimilar available?
A: Not yet. Biosimilars are expected once patents expire (roughly 2028). Reuters+1 -
Q: Do infusion center fees count in my cost?
A: Yes. The drug cost is one component; infusion center or hospital facility fees, monitoring, labs, imaging all add to the total. -
Q: Can a provider negotiate a lower cost for me?
A: Providers and payers may negotiate behind the scenes, but patients rarely directly negotiate drug cost except through assistance programs or insurance appeals. -
Q: Will Keytruda’s list price increase in 2026?
A: Possibly, consistent with inflation adjustments or business strategy, unless policy constraints intervene. -
Q: How often is Keytruda given?
A: Common schedules: 200 mg every 3 weeks, or 400 mg every 6 weeks (depending on cancer type and prescribing practice). keytruda.com+1 -
Q: If my insurance denies coverage, what can I do?
A: You can appeal with medical justification, or seek assistance via Merck’s patient support or nonprofit groups. -
Q: Are there state programs that help with Keytruda costs?
A: Yes — some states have cancer drug assistance or pharmaceutical assistance programs; availability and rules vary by state. -
Q: What documentation is needed for assistance programs?
A: Typically: proof of income, tax returns, prescription, medical records, insurance status, and provider completion of forms. -
Q: Will biosimilars drastically lower cost?
A: Likely yes, in the long term. Competition tends to reduce net prices, though discounts and uptake may vary. -
Q: Can I pay monthly instead of per infusion?
A: Not for the drug itself — payment is tied to each infusion. But some assistance programs may smooth cost over time. -
Q: How do I find out exactly what I will pay?
A: Contact your oncology team, infusion center billing office, and your insurer. Ask for an estimate of drug + facility + ancillary costs. Use support services like Merck’s Access Program to guide you.
Recommendations & Tips for Patients
-
Get cost estimates before treatment begins. Ask your oncologist or infusion center for a “cost estimate” of drug + facility + supporting services.
-
Check your insurance benefit design carefully. Understand whether Keytruda is billed under “medical benefit” or “pharmacy benefit” — the cost sharing (deductible, coinsurance) differs.
-
Enroll in assistance programs early. Some assistance requires that you enroll before first infusion or early in treatment.
-
Use appeal rights. If your insurer denies coverage or requests restrictions, your doctor can help appeal based on medical necessity.
-
Track your out-of-pocket maximum. If you reach your plan’s max, your remaining costs may be covered.
-
Ask for itemized bills. This helps you understand where costs are coming from and catch billing errors.
-
Talk to financial navigators. Many cancer centers have patient navigators or social workers who can help with insurance, charity, and assistance programs.
-
Stay informed about policy changes. With upcoming Medicare negotiation and biosimilar entry, the cost landscape may shift during your treatment.
🆕 Latest Developments to Add
1. FDA Approval of Subcutaneous Version — Keytruda Qlex
-
In September 2025, the FDA approved a subcutaneous (under-the-skin) formulation of Keytruda, branded Keytruda Qlex, combining pembrolizumab + berahyaluronidase alfa. Reuters+1
-
The new version allows administration in as little as 1–2 minutes, compared to ~30 minutes infusion for IV. Reuters
-
Recommended dosing: 395 mg every 3 weeks or 790 mg every 6 weeks. Reuters
-
Merck expects that 30–40% of patients may transition to the subcutaneous version within a couple of years, due to convenience and site-of-care flexibility. biopharmadive.com+1
-
Pricing strategy: Merck plans to price Qlex at parity with Keytruda IV in many cases, to maintain market share. biopharmadive.com
Implication to your article:
-
Add a section on “Emerging Subcutaneous Option & Market Impact”.
-
Discuss how the new form could affect costs, infusion center fees, site-of-care choices, and insurance billing dynamics.
-
Note that payers may prefer the subcutaneous format due to lower chair time and infusion costs. biopharmadive.com
2. Merck & Market Strategy Amid Patent & Price Pressure
-
Merck is preparing for patent losses in 2028 and the entrance of biosimilar competition. Reuters+3fiercepharma.com+3biopharmadive.com+3
-
To offset revenue risks, the company is cutting costs ($3 billion in internal cost reduction) and shifting resources to newer drugs and R&D. biopharmadive.com+1
-
Merck expects Keytruda to be one of the drugs subject to Medicare price negotiation under the Inflation Reduction Act, with new negotiated pricing effective as of January 1, 2028. Reuters+2fiercepharma.com+2
-
However, recent changes in U.S. tax and budget law broaden the orphan drug exclusion, which may delay Keytruda’s selection for price negotiation by at least one year. KFF+1
-
Specifically: under the new law, the orphan drug exclusion’s scope is expanded to delay eligibility for negotiation for drugs like Keytruda. KFF
Implication for your article:
-
In the policy/regulation section, insert these updates showing shifting legal context on negotiation eligibility.
-
Emphasize uncertainty: originally anticipated negotiation in 2026, but changes may push selection to 2027, with pricing change in 2029. KFF+1
-
Highlight how the combination of patent expiry, biosimilar threat, and negotiation risk will likely drive pricing pressure and competitive strategies.
3. Commercial Insurance Patient Cost Trends (Merck Data)
-
According to Merck’s Keytruda “Cost, Insurance & Financial Help” page (updated as of September 2025), for patients with commercial/private insurance receiving a 200 mg dose:
-
59% of patients paid no out-of-pocket cost for that infusion. keytruda.com
-
For those who did have OOP costs, ~80% paid between $0.01 and $375 per infusion (after deductibles) under their plan. keytruda.com
-
-
The same page reaffirms list price (WAC) for 3-week dose = $12,031.36 and 6-week dose = $24,062.72 (as of Sept 2025). keytruda.com
Implication to your article:
-
Revise the table of “commercial / private insurance” out-of-pocket ranges with this updated data: many patients pay zero OOP; those who don’t pay often under $375 per infusion.
-
Use Merck’s page as a primary reference / link.
4. Spending, Market Share & Drug Utilization
-
In 2023, Medicare + beneficiaries spent $5.6 billion on Keytruda (it was the top-spend drug under Medicare Part B) KFF
-
As of 2024, Keytruda generated $29.48 billion in U.S. sales, representing ~46% of Merck’s total sales. Reuters+1
-
The expectation is that sales will decline post-negotiation and post-patent expiry. Reuters+2fiercepharma.com+2
Implication to your article:
-
Use those high sales numbers in your market pressure / context section to show the financial stakes.
-
Emphasize that the scale of spending is what makes Keytruda a target in policy and negotiation.
Suggested Insertions / Edits in Your Article
-
In your “Regulatory & Policy Developments” section:
“In September 2025, Merck gained FDA approval for a subcutaneous formulation (Keytruda Qlex), which may shift costs, site-of-care dynamics, and payer preferences. Reuters+1
While Keytruda was previously expected to be selected for Medicare negotiation in 2026, changes to the orphan drug exclusion law may delay its selection until 2027, with negotiated pricing taking effect in 2029. KFF+1” -
In your “What Patients Actually Pay” / Commercial Insurance table or narrative: update out-of-pocket ranges per Merck: many pay $0 OOP; for others, up to $375. keytruda.com
-
Add a “Subcutaneous Option & Cost Implications” subheading:
“The advent of Keytruda Qlex (SC) may reduce infusion center costs, improve convenience, and reshape patient billing. Merck intends to price it close to existing IV per-infusion costs. biopharmadive.com+2Reuters+2”
-
In “Forecast & Trends”: mention Merck’s internal cost cuts and strategy shifts ahead of patent loss. biopharmadive.com+1
-
In “Comparison vs alternatives / biosimilars”: mention that biosimilar development and deals are underway (e.g. Dr. Reddy’s / Alvotech). The Economic Times
-
Add relevant external links / citations:
-
Keytruda cost & support page — “Cost, Insurance & Financial Help” keytruda.com
-
Reuters article on Merck expecting Keytruda in negotiation list Reuters
-
Medical News Today updated cost overview medicalnewstoday.com
-
Articles on orphan drug exclusion / negotiation delay KFF+2petrieflom.law.harvard.edu+2
-
News on Keytruda Qlex approval Reuters+2Wikipédia+2
-






Add a Comment