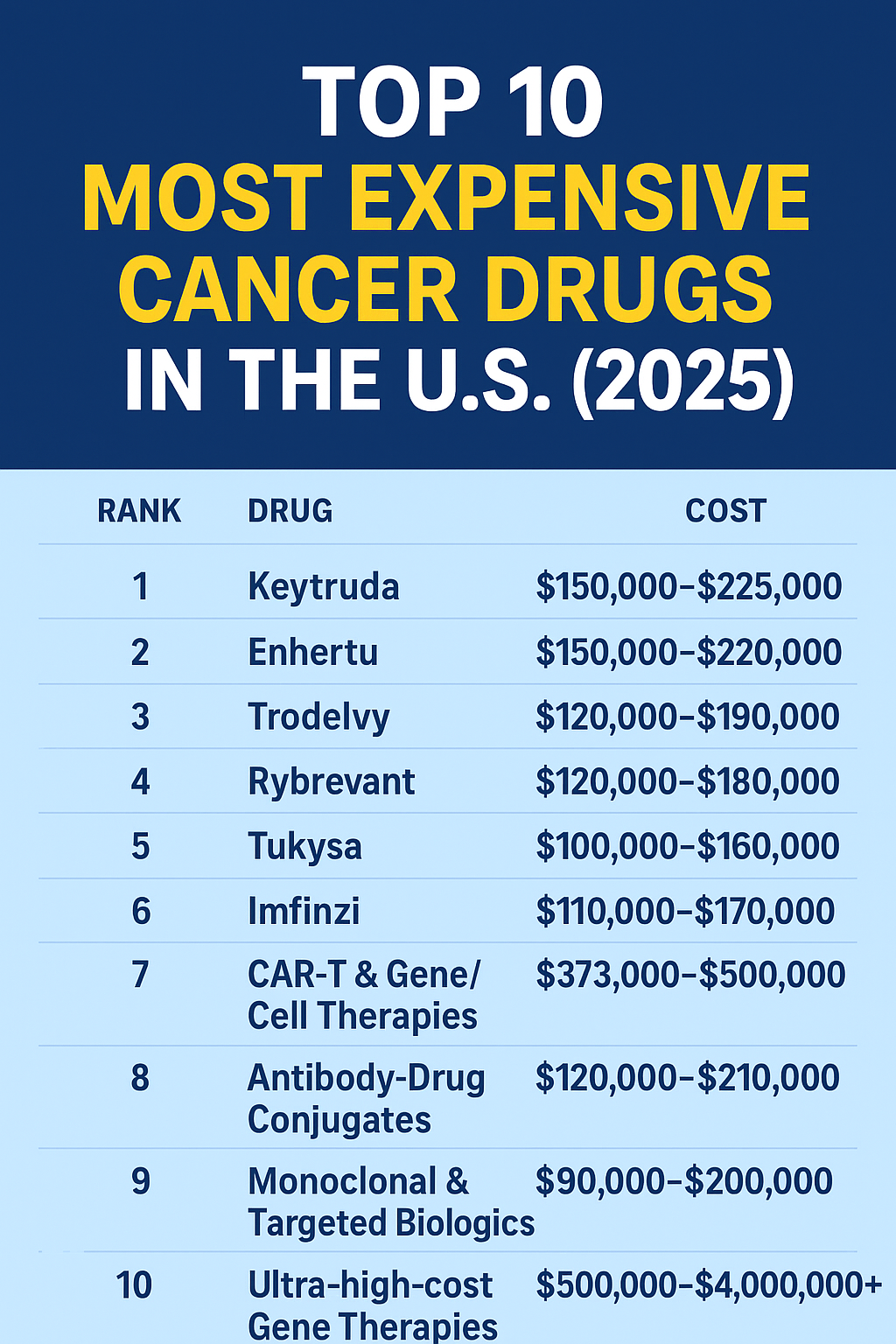
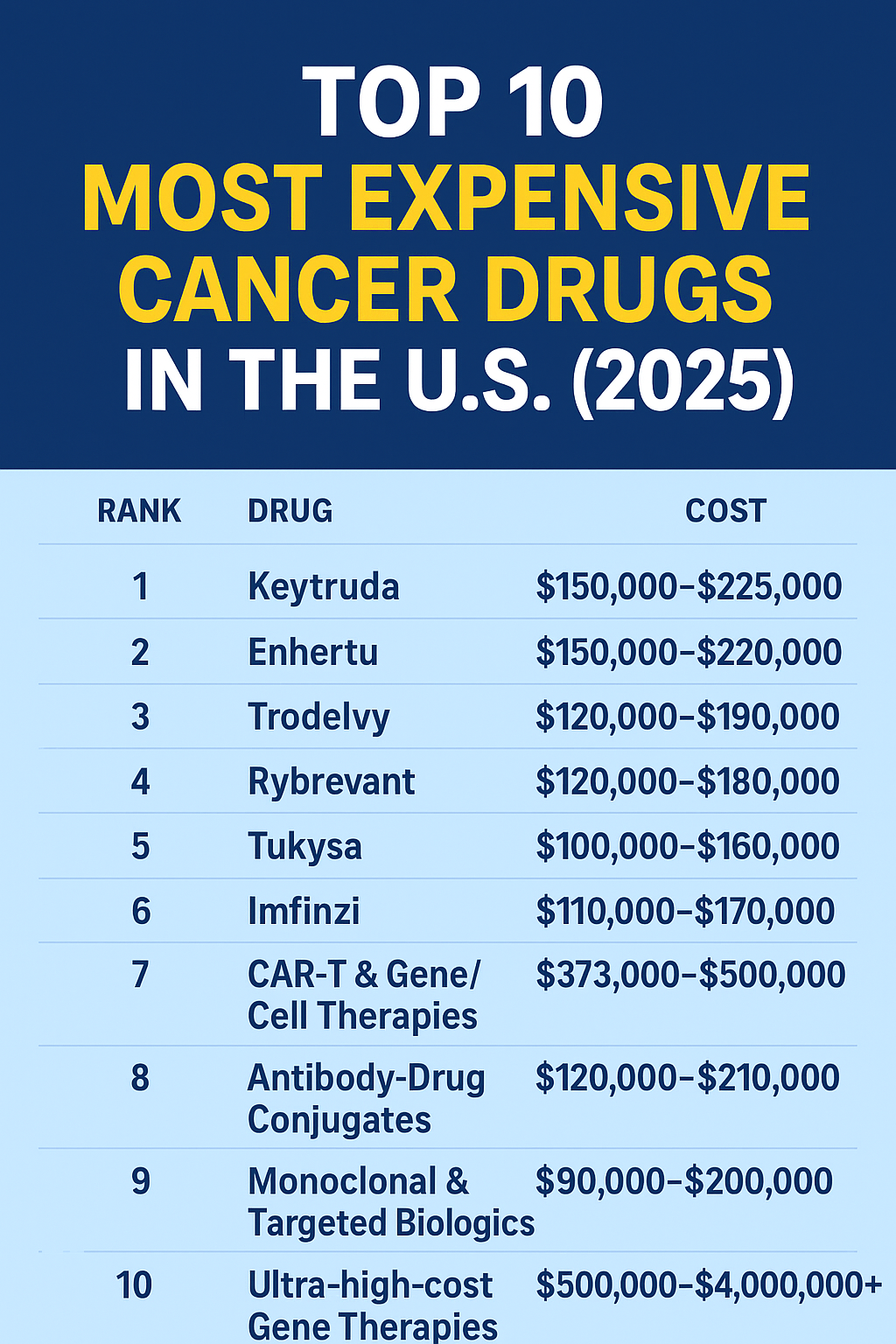
Top 10 most expensive cancer drugs in the U.S. — 2025 (estimated annual cost)
Notes: “Estimated annual cost” is a practical metric. For single-administration gene therapies we show per-patient treatment cost. All figures are rounded ranges and reflect list or public pricing references where available; actual payer reimbursements and patient out-of-pocket amounts vary widely.
| # | Drug (generic) | Indication / Class | Manufacturer | Estimated annual cost (USD) | Notes & sources |
|---|---|---|---|---|---|
| 1 | Keytruda (pembrolizumab) | PD-1 immune checkpoint inhibitor — multiple cancers | Merck | $150,000 – $225,000 per year (typical IV regimens; ASP-based estimates ~$11,700 per 200 mg dose, q3w; listed 6-week dose ~$23,590.88). | High use & list prices: Merck pricing page; Medicare ASP benchmark (ASP $58.562/mg). :contentReference[oaicite:0]{index=0} |
| 2 | Enhertu (trastuzumab deruxtecan) | HER2-targeted antibody-drug conjugate — breast, gastric cancers | AstraZeneca / Daiichi | $150,000 – $220,000 per year (varies by dosing & indication) | ADCs often have high per-cycle costs and long treatment durations; manufacturer pricing and payer reviews reflect large annual spend. (See market reporting & oncology pricing reviews). :contentReference[oaicite:1]{index=1} |
| 3 | Trodelvy (sacituzumab govitecan) | ADC for breast, urothelial cancers | Gilead Sciences | $120,000 – $190,000 per year | High cycle cost and chronic administration for responders; clinical use and cost-effectiveness papers document large annual expenditure. :contentReference[oaicite:2]{index=2} |
| 4 | Rybrevant (amivantamab) | EGFR/MET bispecific antibody — NSCLC | Johnson & Johnson / Janssen | $120,000 – $180,000 per year | New targeted biologics frequently command high per-cycle prices; see industry pricing analyses. :contentReference[oaicite:3]{index=3} |
| 5 | Tukysa (tucatinib) | Oral HER2-directed kinase inhibitor — breast cancer | Seagen / Gilead | $100,000 – $160,000 per year | Oral targeted therapies can be used long-term, driving annual cost even when per-pill price seems moderate. :contentReference[oaicite:4]{index=4} |
| 6 | Imfinzi (durvalumab) | PD-L1 inhibitor — lung and other cancers | AstraZeneca | $110,000 – $170,000 per year | Another high-use checkpoint inhibitor with costs similar to other PD-1/PD-L1 drugs. Pricing and Medicare reimbursement reported in public sources. :contentReference[oaicite:5]{index=5} |
| 7 | CAR-T & Gene/Cell Therapies (selected oncology examples) | One-time engineered cell therapies (e.g., Breyanzi, Yescarta variants) | Gilead, BMS, others | $373,000 – $500,000 per treatment (therapy-specific) | CAR-T therapies carry high single-treatment prices reflecting manufacturing complexity; hospital & site costs add substantial amounts. Industry lists and payer reports show high per-patient costs. :contentReference[oaicite:6]{index=6} |
| 8 | Antibody-Drug Conjugates (other examples) | Various ADCs (e.g., polatuzumab, enfortumab vedotin) | Roche, Seagen, Astellas | $120,000 – $210,000 per year (depending on indication & cycles) | ADCs are a fast-growing, high-cost oncology class; costs reflect drug payload + antibody complexity. :contentReference[oaicite:7]{index=7} |
| 9 | Monoclonal & Targeted Biologics (selected) | Various targeted mAbs (e.g., Perjeta, Ibrance in oncologic combos) | Roche, Pfizer, others | $90,000 – $200,000 per year (depending on combination regimens) | Many oncology biologics reach high annual costs when used long-term or in combination. Reference: industry sales reports and cost-effectiveness literature. :contentReference[oaicite:8]{index=8} |
| 10 | Ultra-high-cost Gene Therapies (oncology/rare overlaps) | Single-administration therapies & rare-disease oncology overlaps | Various | $500,000 – $4,000,000+ per treatment (therapy-dependent) | Although many of the absolute most expensive medicines are gene therapies for rare conditions (Zolgensma, Hemgenix, etc.), some oncology or rare tumor gene therapies also approach these ranges; see 2025 expensive drug roundups. :contentReference[oaicite:9]{index=9} |
Important: this list mixes categories (chronic immunotherapies vs. single-admin gene/cell therapies) because both types dominate the upper end of oncology spending in 2025. For a patient, the relevant figure is not just list price but allowed amount, site-of-care charges, administration fees, and insurer cost-sharing.
How we estimated costs (method)
- We used manufacturer public list prices when available (example: Merck’s published 6-week list price for Keytruda) and Medicare ASP per-mg rates to compute typical administration costs. :contentReference[oaicite:10]{index=10}
- For CAR-T and gene therapies we used reported launch prices and recent market lists showing million-dollar treatments. :contentReference[oaicite:11]{index=11}
- We cross-checked industry reporting and peer-reviewed articles on oncology drug pricing and utilization to normalize annual cost ranges. :contentReference[oaicite:12]{index=12}
What patients actually pay (practical guidance)
- Insurance matters most: private plans, Medicare Part B, Medicare Advantage and Medicaid treat high-cost oncology drugs differently. Medicare Part B often reimburses infused drugs with patient coinsurance around 20% unless secondary coverage exists. :contentReference[oaicite:13]{index=13}
- Site-of-care fees: hospital outpatient departments commonly bill facility fees and infusion administration charges that add thousands to final bills.
- Manufacturer support: co-pay assistance and patient programs are available for many brand oncology therapies but have eligibility caps and rules—always verify current programs. :contentReference[oaicite:14]{index=14}
- Ask for estimates: request a predetermination from your insurer and an itemized estimate from the infusion clinic to understand expected out-of-pocket cost before starting therapy.
Policy changes & 2025 outlook
Policy shifts matter: the Inflation Reduction Act price negotiation process and Medicare negotiation timelines are affecting pricing strategy for top-selling drugs (Merck expects Keytruda could be part of negotiation frameworks). Patent expirations (notably Keytruda around 2028) and the arrival of biosimilars/biosimilars-like competitors will influence list prices and payer bargaining going forward. :contentReference[oaicite:15]{index=15}
Internal links & further reading (suggested)
- Keytruda Cost in the USA (2025 Update)
- Opdivo Cost in 2025: Nivolumab Price & Insurance
- Guide to Manufacturer Assistance Programs (Cancer Drugs)
FAQs
Which cancer drug is the most expensive?
If we measure by per-treatment cost, certain gene therapies and rare-disease biologics top the list (prices in the millions). For chronic cancer drugs used by many patients, immunotherapies and ADCs top annual spending (often $100k–$400k/year). :contentReference[oaicite:16]{index=16}
Will prices fall soon?
Some downward pressure is expected from biosimilars, patent expiries, and government negotiation programs—but changes will vary by drug class and market. :contentReference[oaicite:17]{index=17}
How can patients lower costs?
Check insurance coverage, apply for manufacturer assistance, consider alternate sites of care (when clinically appropriate), and discuss clinical trial options with your oncologist.
Sources & further reading
- Keytruda — Cost, Insurance & Financial Support (Merck). :contentReference[oaicite:18]{index=18}
- Reuters — FDA approves Keytruda subcutaneous version (Sept 2025). :contentReference[oaicite:19]{index=19}
- FiercePharma — Most expensive drugs in the US (2025 overview). :contentReference[oaicite:20]{index=20}
- SerifHealth — Medicare ASP numbers & Keytruda ASP math. :contentReference[oaicite:21]{index=21}
- Peer-reviewed review — High costs of anticancer therapies (PMC). :contentReference[oaicite:22]{index=22}
- RazorMetrics — Most expensive drugs 2025 (context on gene therapies). :contentReference[oaicite:23]{index=23}
Published: September 24, 2025 — This article summarizes public pricing information and industry reporting. Prices change frequently; always verify current manufacturer lists, insurer estimates and clinic billing for patient-specific cost information.







Add a Comment